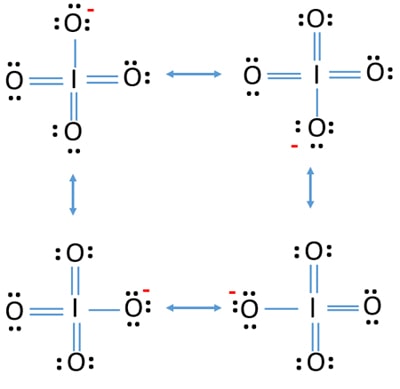
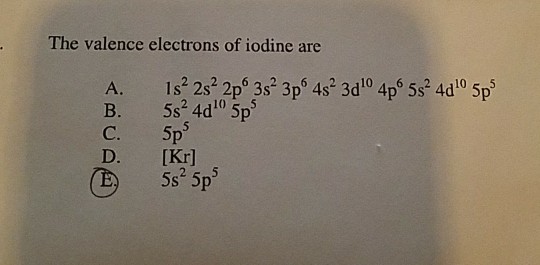Iodine valence electrons - I3
Periodic Table of Elements: Iodine
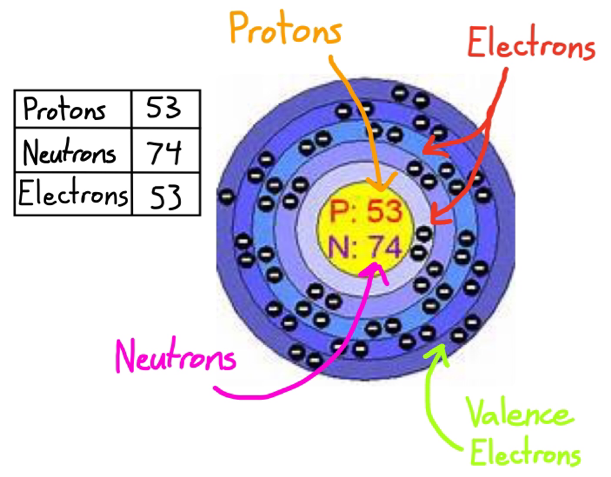
During the formation of bonds, the last shell of iodine receives an electron and turns into an iodide ion I —.
LiF is almost insoluble in water whereas LiCl soluble not only in water but also in acetone.
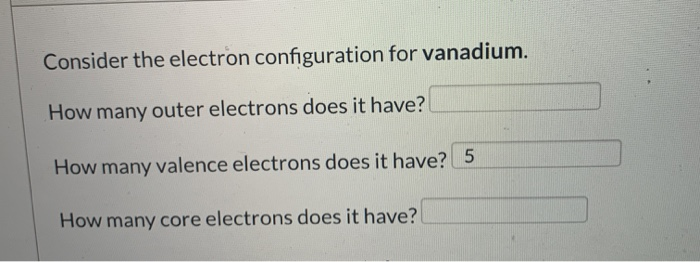
Iodine is in the group 17 , known as halogens, and all of them have 7.
- Related articles
2022 blog.dabchy.com