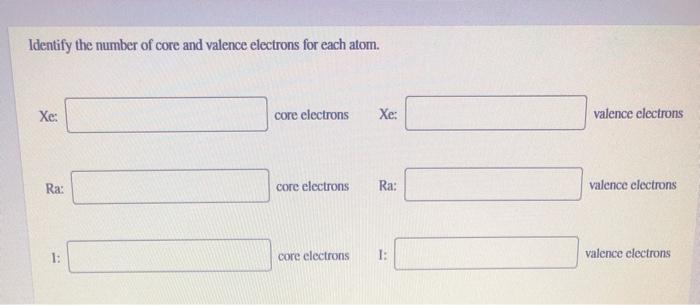
Xe valence electrons - How many valence electrons are in Xe2+ class 11 chemistry CBSE
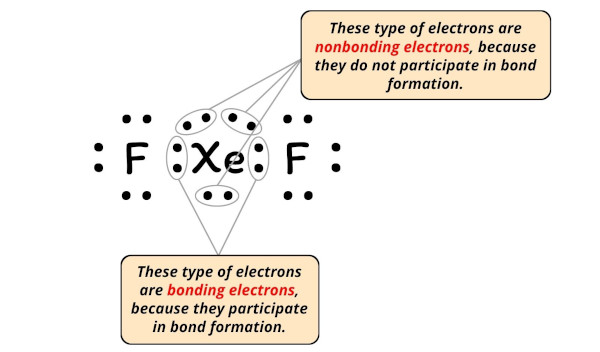
xef2 lewis structure homework Help at TutorEye
The chemical properties of the atom are determined by the number of protons, in fact, by number and arrangement of electrons.
The complete idea of the orbit is given there.
Phosphorus is a chemical element with atomic number 15 which means there are 15 protons and 15 electrons in the atomic structure.
valence electron configuration
Germanium is a chemical element with atomic number 32 which means there are 32 protons and 32 electrons in the atomic structure.
The electrons in the highest numbered subshells are the valence electrons which comprise the valence shell of the atom.
The outermost shell of the fluorine F atom possesses seven electrons, and its valency might be seven, but it is easier for it to gain one electron than to lose seven.
- Related articles
2022 blog.dabchy.com