Weak acid - Why is HF a weak acid?
Weak Acids & Bases
It can react as an oxidising or reducing agent, which means that its nitrogen atom can gain or lose electrons in reactions with other substances.
It should also be noted that solid acetic acid is known to have hydrogen bonding.
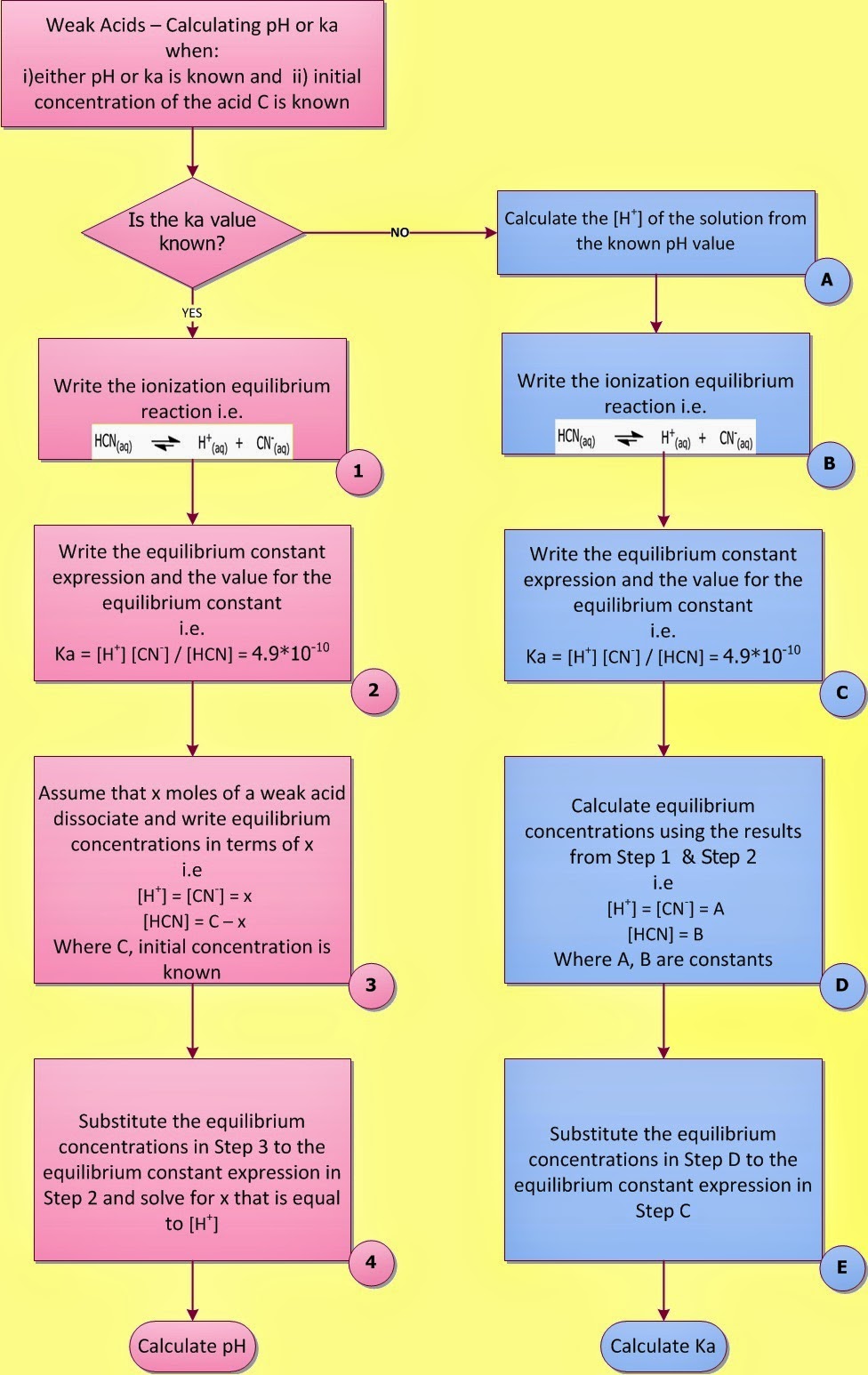
The equilibrium constant for an acid is called the acid-ionization constant, K a.
Why is HF a weak acid?
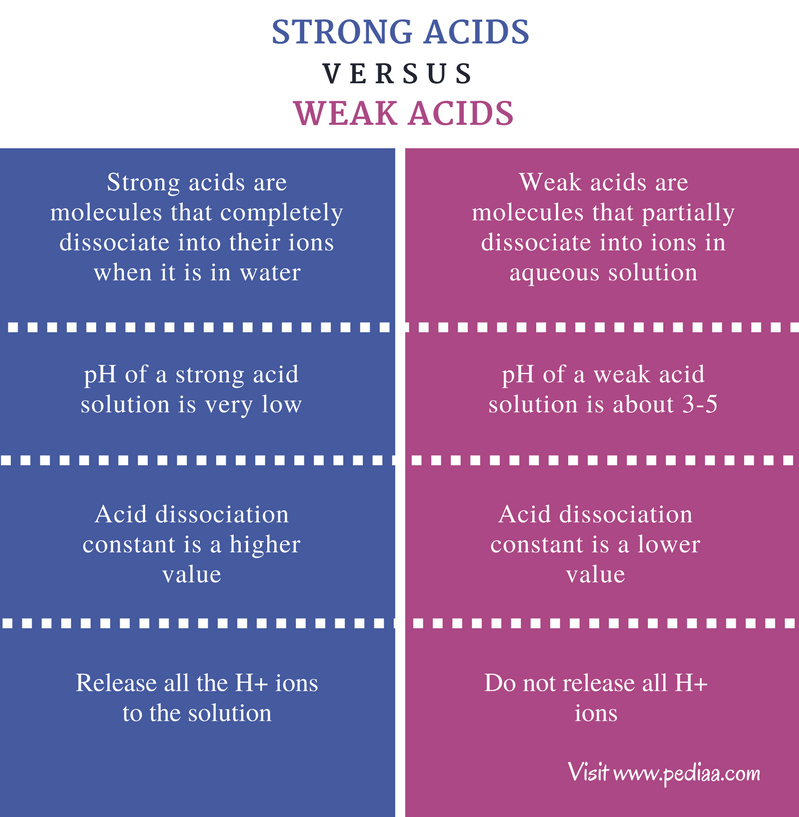
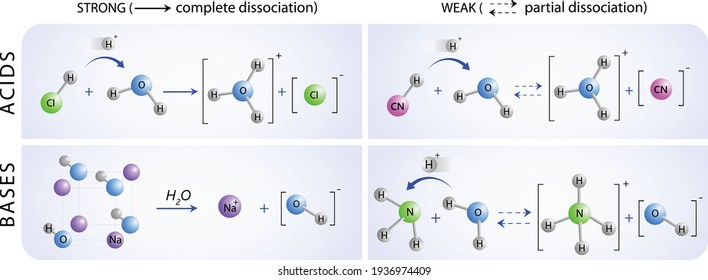
Such substances release H ions in solution but not very readily and are called weak acids.
Concentrated and dilute acids Weak and strong should not be mistaken for dilute and concentrated.
It can quickly ionise or dissociate in a solution.
- Related articles
2022 blog.dabchy.com
/list-of-strong-and-weak-acids-603642-v2copy2-5b47abd0c9e77c001a395e55.png)