Partial pressure formula - Dalton's Law of Partial Pressure
Recent Posts
- Bellyfly testimoni
- Louis wain
- Senarai atlet malaysia olimpik 2021
- Cara login rhb online banking
- Hiro hamada
- Russia vs finland prediction
- Best tracking number
- Portal rasmi pdrm
- Indonesia population
- Minyak tanah in english
- Tin price today
- Kembara seniman jalanan
- Amin idris dan isteri
- Ramos spain
- Tiktok downloader with watermark
- Netherlands vs czech republic h2h
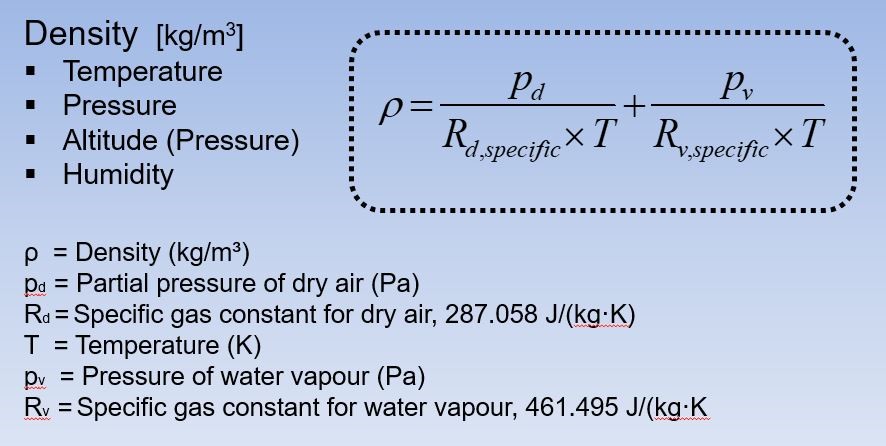
Dalton's Law of Partial Pressure
In ideal gas molecules are very far from each other so they do not react.
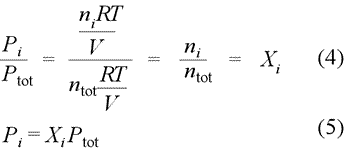
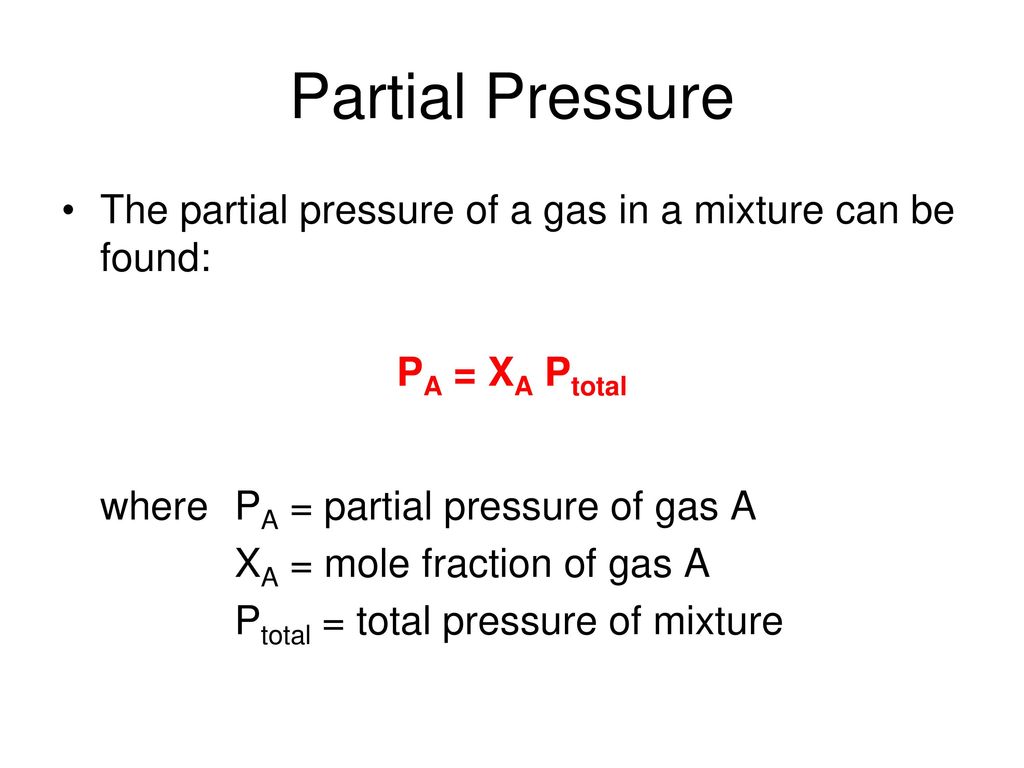
The partial pressure of an individual gas is equal to the total pressure multiplied by the mole fraction of that gas.
In chemistry mole fraction is the ratio of a particular gas component in a mixture and the total number of moles of all constituents in the mixture.
- Related articles
2022 blog.dabchy.com