The emission line spectrum of hydrogen derives from the energies released when - Optical Spectroscopy of Hydrogen and Deuterium
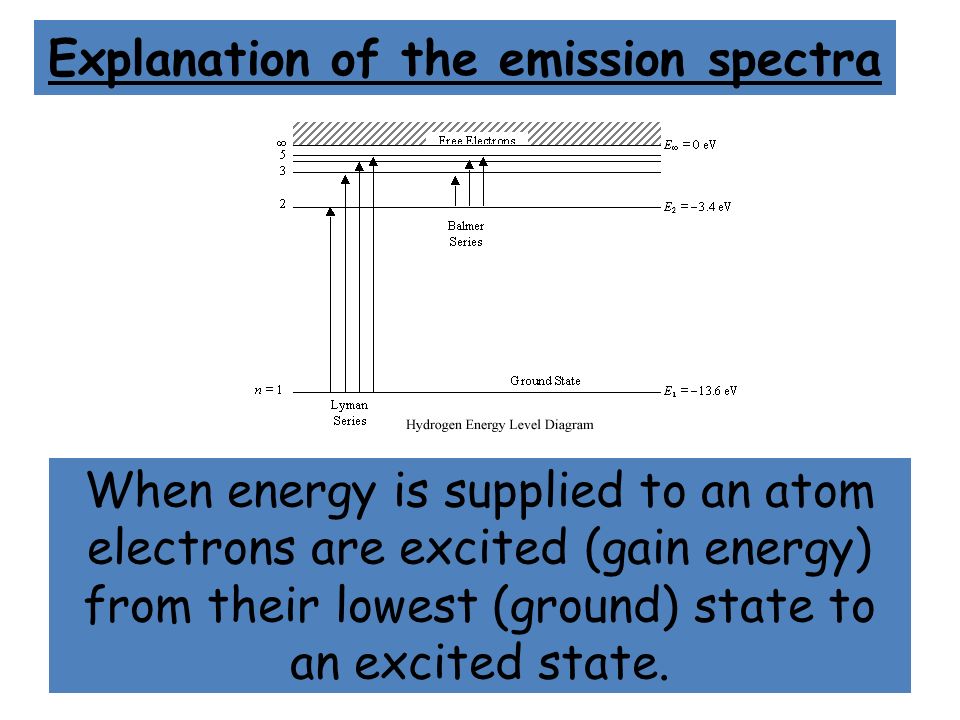
6. The line emission spectrum of hydrogen provides evidence for the existence of electrons in discrete energy levels, which converge at higher energies.
The colors of hydrogen
Spectral Series: Hydrogen Spectrum, Rydberg Formula
Rydberg Formula: Equation & Sample Questions
Spectrum of Hydrogen Atom
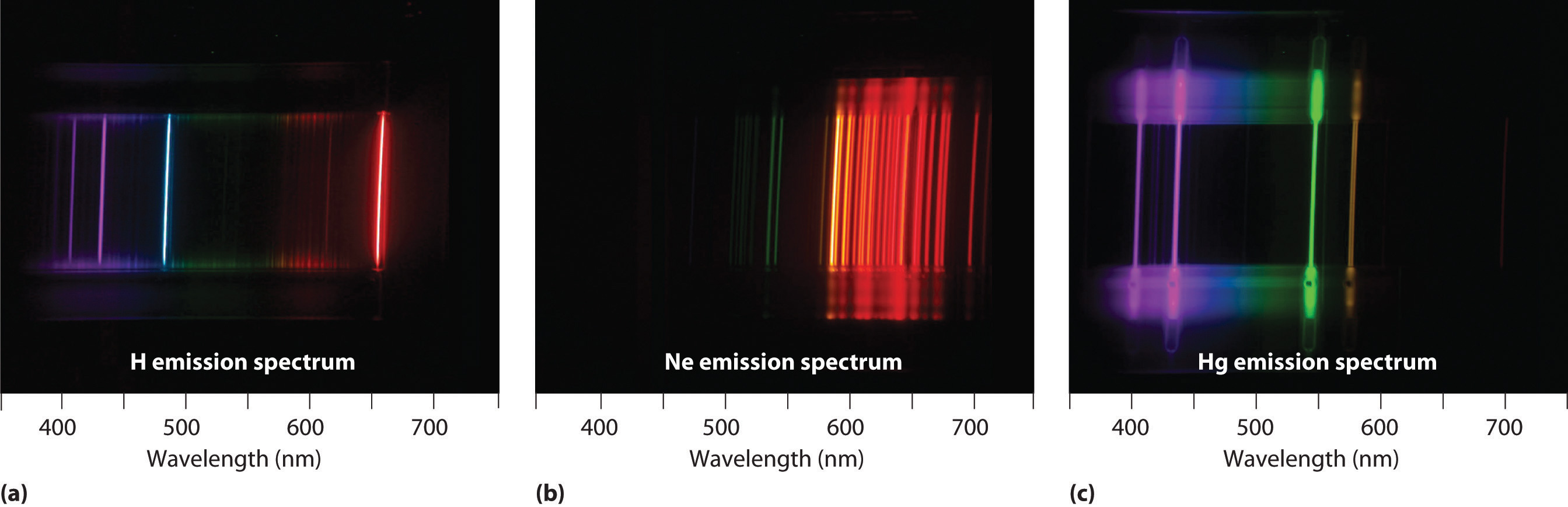
Why does helium have more emission lines than hydrogen?
Atomic Emission Spectra; Hydrogen Emission Spectrum
Erwin was taught at home, by tutors and parents, until he was 11.
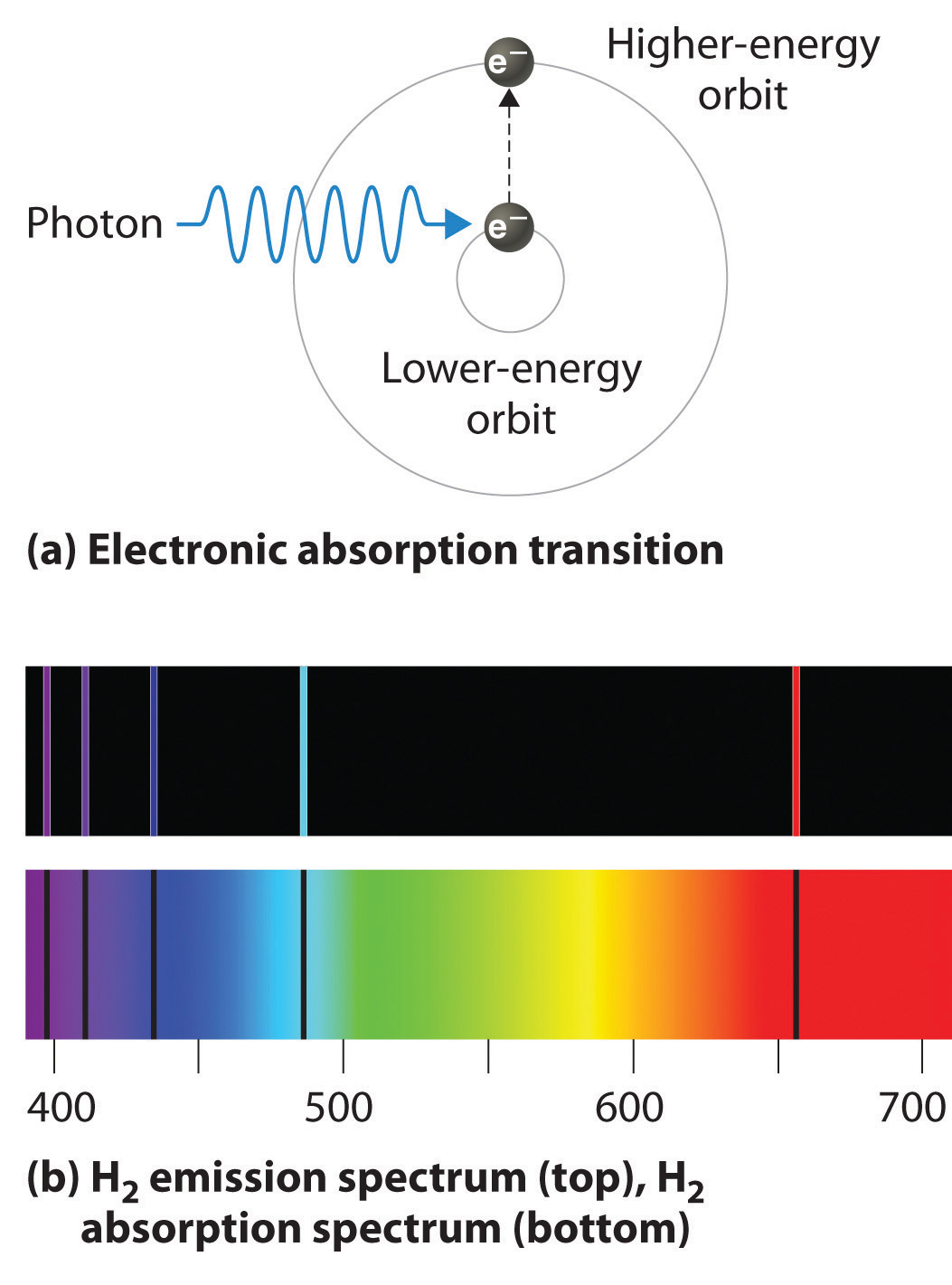
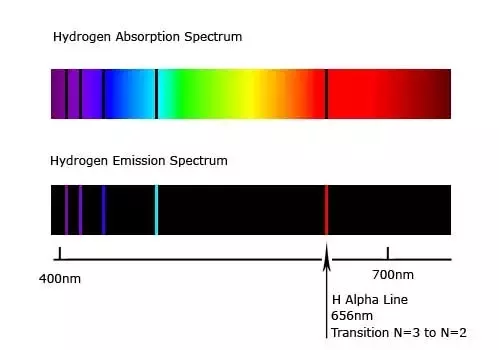
You'll also see a blue green line and so this has a wave length of 486 nanometers.
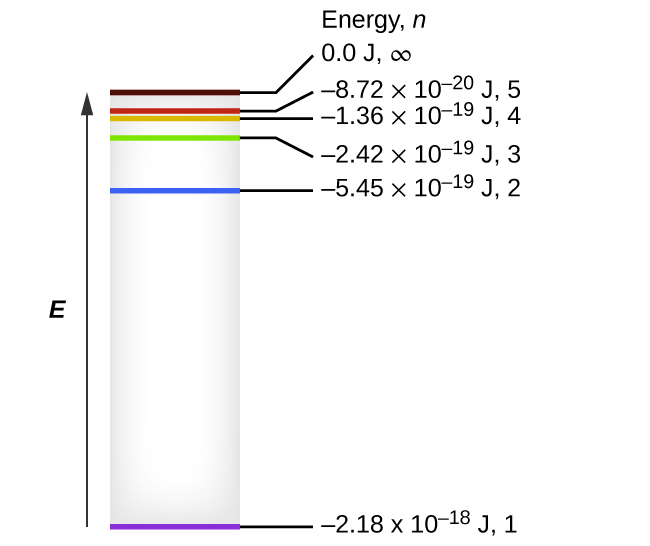
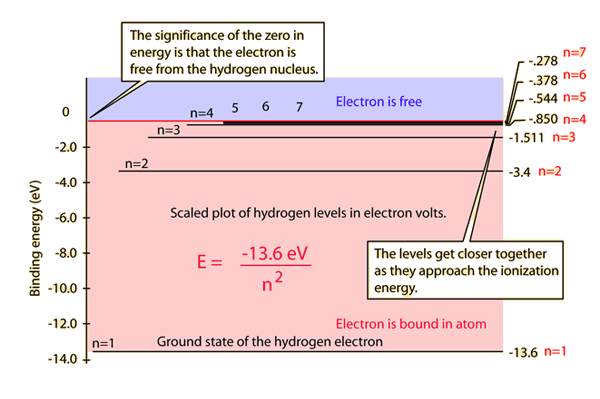
Thus the energy levels of a hydrogen atom had to be quantized; in other words, only states that had certain values of energy were possible, or allowed.
Hydrogen energies and spectrum
Further series for hydrogen as well as other elements were discovered as spectroscopy techniques developed.
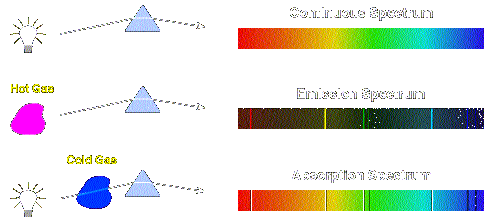
With sodium, however, we observe a yellow color because the most intense lines in its spectrum are in the yellow portion of the spectrum, at about 589 nm.
So when you look at the line spectrum of hydrogen, it's kind of like you're seeing energy levels.
- Related articles
2022 blog.dabchy.com

















:max_bytes(150000):strip_icc()/OlympicPool-5c28632046e0fb0001193055.jpg)